Validation (IQ/OQ/PQ) Simplified
Acronyms are commonplace in everyday life. If someone mentions the DMV, IRS, or FBI, or a form asks you for your DOB or SSN, you know exactly what that person is talking about. The manufacturing industry is no different, so if you hear people mention IT, FTP, DFM, and EC, you typically know exactly what is being referenced.
Occasionally, we meet up with alphabet soup that is unfamiliar to us, or if we have heard it, we don’t quite understand everything that it encompasses. For those involved in the medical or pharmaceutical industries, you have probably heard about process validation and the acronyms IQ, OQ, and PQ. These terms can often be seen when receiving a proposal from your molding supplier, or when you are asking about the status of your molding tools.
Understanding validation and why it shows up in many FDA and Health Canada guidance documents is critical. For industries involved in health care equipment, product quality is paramount, and minute inconsistencies can have disastrous consequences.
A successfully validated process is stable, dimensionally centered with the required tolerance, and minimizes rejects with significantly fewer adjustments from build to build. Early supplier involvement (ESI) is especially important as Design for Manufacturing (DFM) interaction can quickly identify and resolve issues like dimensional tolerance, molding techniques required, and the approval and acceptance criteria.
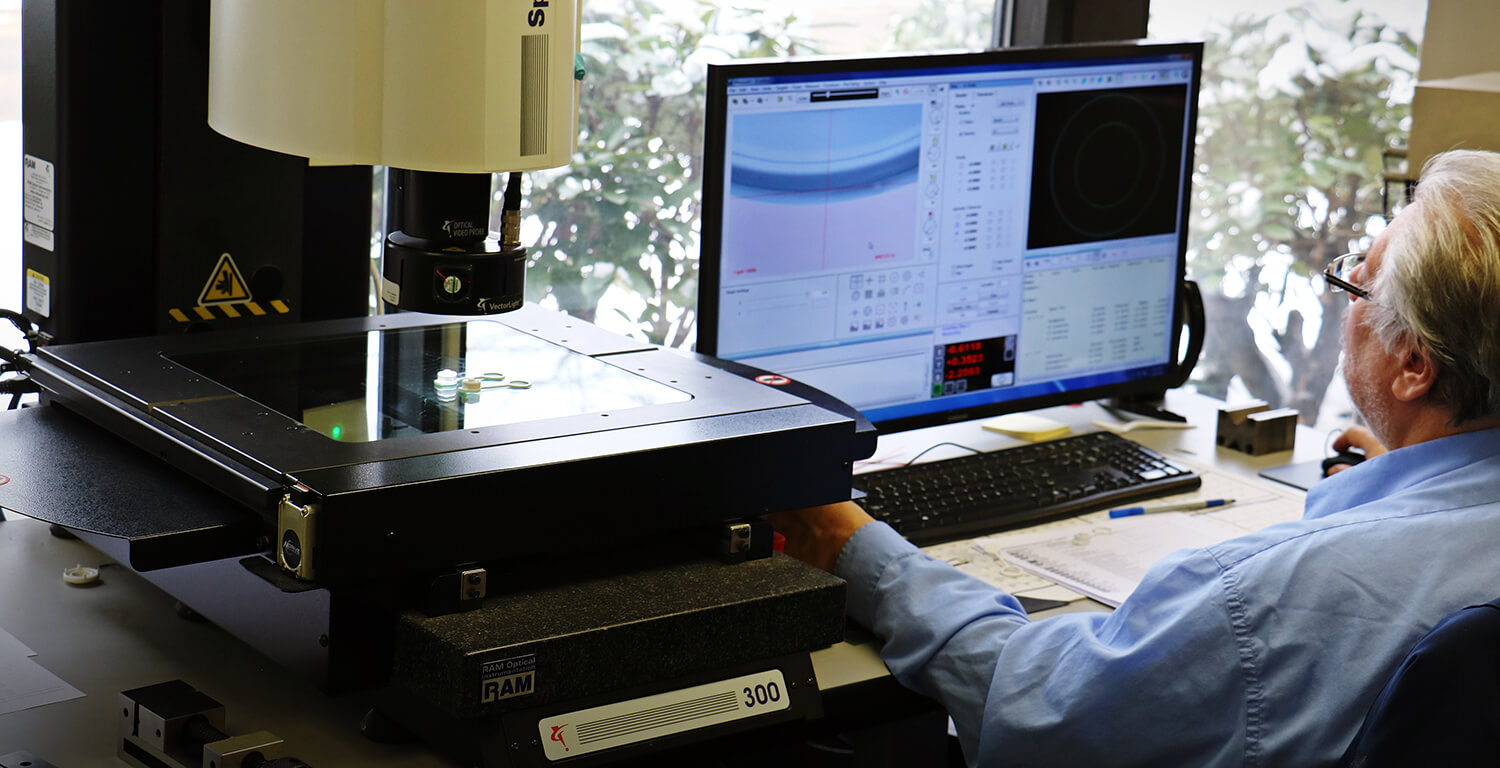
Installation Qualification (IQ)
IQ, or Installation Qualification, is the first step in the validation process and focuses on stable equipment and processes. The FDA definition of installation qualification is: “Establish confidence that process equipment and ancillary systems are compliant with appropriate codes and approved design intentions, and that the manufacturer recommendations are suitably considered.”
In basic terms, the installation qualification is an executed test protocol that verifies that a system has the required prerequisite conditions to function as designed. This involves focusing on:
- Equipment selection and material
- Equipment functionality and main features
- Verification and test that the press and mold are operating within desired parameters
IQ provides the opportunity to benchmark specific processes and molding conditions that will impact part consistency and stability over the life of the program. During the IQ process, a precision molder can confirm that there is dimensional stability, and if not, what specific issues may be the root cause. This could be attributed to barrel or screw size, temperature control, water flow, proper mold fill, or any number of equipment or material variables. Documenting the initial installation settings allows for quick identification of the root cause of rejects.
Operational Qualification (OQ)
The next phase is OQ, or Operational Qualification. This is at the center of evaluating and defining the injection molding process. In this phase, you want to verify that your manufacturing process is achieving the desired operational requirements. This involves engineering studies, statistical and dimensional evaluations, and leveraging analytical processes. The goal is to determine the appropriate operating limits of various process parameters to ensure that you can repeatedly meet the requirements of the end product.
The process settings selected for the OQ are developed following industry-standard scientific molding processing procedures utilizing decoupled II guidelines. This establishes an acceptable process range in which manufactured components can meet all required specifications as described in the drawing and customer requirement documents.
Once the process range is established, test runs are then conducted, including a 30-piece capability study for each Critical to Function and Critical to Process dimension is identified. A full first article inspection (FAI) of three pieces is performed so that all samples can be visibly inspected for any cosmetic issues (flash, sink, short shots, splay, etc.), and complete attribute checks are performed on the collected samples per the drawing and control plan. If the parts do not meet the high and low run target Cpk and Ppk values that have been determined by the customer’s statistical requirements, the process parameters can be narrowed or revisions to the print specifications can be made to account for the actual result of the molded parts.
By going through the scientific molding experiment, which is supported by data, the molder is more likely to identify issues that arise in a production setting to produce more favorable parts dimensionally and cosmetically all while compressing lead times.
Performance Qualification (PQ)
The goal of the Performance Qualification, or PQ phase, is to prove that the process derived in OQ can withstand lot, setup, and operator variation, among others. The PQ is to demonstrate that the process, under anticipated production conditions, will consistently and repeatedly produce a product that will meet all predetermined requirements for functionality and safety.
Typically, a minimum of 30 pieces from each cavity (if applicable) are randomly collected during each of the 3 PQ runs for inspection. Samples are collected once the molding process is stable. Each run duration is typically set for a minimum of 4 hours. And a full FAI on three pieces from each run is conducted as well. If the PQ is unsuccessful, the root cause is determined, and the molder and customer will collaborate to work toward an acceptable resolution.
Validation Will Give You Peace of Mind
Why does the medical equipment manufacturing industry need this qualification process? A well-planned and performed validation process is critical to confirm that your molding partner has a well-controlled manufacturing solution that can consistently produce products to quality standards.
If a manufacturer fails to demonstrate repeatability, the results can be disastrous. It can potentially jeopardize the safety of the end user, cost the client hundreds of thousands of dollars, increase the risk of product recall, and potentially contribute to a loss of market share. Forum Plastics minimizes those potential risks by communicating change and ensuring that we account for it in our day to day operations. Over the long run, the upfront investment in validation can lead to significant cost avoidance and boost overall quality performance.
All of Forum’s validation activities follow ISO 13485:2016 requirements. Forum offers a complete Validation and Production Part Approval Process (PPAP) suite that can be tailored specifically to your requirements. Forum provides services to help draft validation protocol and has hundreds of previously completed validations to assist our customers in determining the necessary content for each protocol to align with a customer’s specific quality requirements.
Contact Jose Mosello, Director of Quality, or Andrew Butkus, Engineering and Metrology Manager, for more information.
Stay Connected
Sign up to get updates from Forum