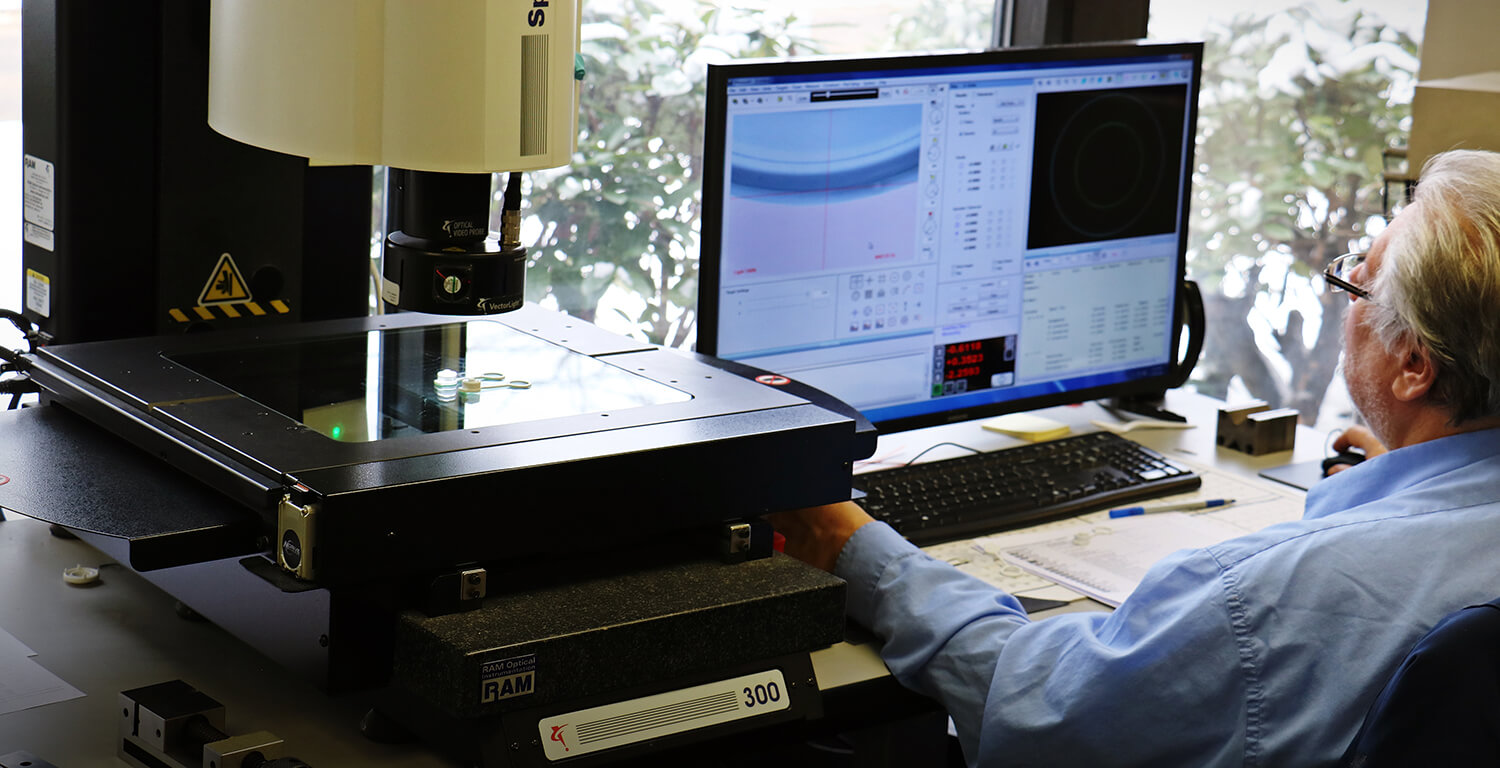
Forum is committed to maintaining the highest levels of quality, traceability, and consistency in everything we do.
Forum is an ISO 13485:2016 certified business. Whether your application is Medical or Industrial, all of our manufactured products go through the same quality systems which can be tailored to specific customer requirements. We have the latest equipment, including an industrial CT scanner, and experienced technicians to perform various levels of qualification.

Our Three Step Process
1 Review
Forum can begin a component metrology assessment with only a component sample and material identification. Working with our client, we better understand the design considerations and goals, material & performance requirements and any limitations or critical performance objectives. This information provides the basis for fixture design, algorithm development and repeatable process establishment to optimize data collection and subsequent analysis.
2 Measure
Combining data, analytics and best practices, Forum uses various measurement tools and procedures to determine dimensional stability, gain precision, enhance accuracy, and mitigate uncertainty.
- Example of Measurement Methods/Tools
- Calipers & Gauges
- Coordinate Measuring Machine (CMM)
- CT Scanner Services
- Dial Indicators
- Optical Comparator
- Micrometers
- Surface Plates
- Vision Measuring Machine (VMM)
3 Validate
Following the measurement process, validation is performed by analyzing factors critical to the design and manufacturing repeatability of the component. Full validation protocol is determined based upon mutual agreement and client requirements.
As part of our validation process, Forum provides clients with detailed information to best support and inform their design and subsequent quality, regulatory and market-based strategies.
Analysis Assessment Examples
- Coordinate Measurements
- Inclusions
- Manufacturing Method
- Material Constitution
- Nominal/actual
- Porosity
- Wall Thickness

Our Quality and Metrology Lab Environment
Our Quality and Metrology Lab environment is fully air-conditioned and monitored. The well-apportioned and brightly lit lab is operated and fully staffed across the three shifts of manufacturing operations. Our electronic records are backed up in an off-site vault for protection and we maintain quality records and production retain run samples to complete our history files. Our lab has extensive electronic measurement equipment and a well-trained and experienced staff.

Case Study:
Forum Uses CT Scanner To Aid Medical Device Company Maintain Supply Chain
Within the medical device industry, many companies get their start by developing a single device or product line, and as this device becomes more and more mainstream, larger companies become interested in adding that device to their portfolio.